Education: Activities & Documents
Properties of Fresh & Sea Water
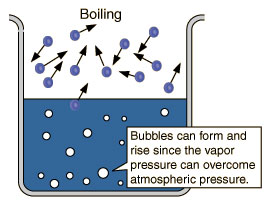
Liquid water (H2O) is often perceived to be pretty ordinary as it is transparent, odorless, tasteless and ubiquitous. Water is unique in that it is the only natural substance that is found in all three states - liquid, solid (ice), and gas (steam) - at the temperatures normally found on Earth. Earth's water is constantly interacting, changing, and in movement. Water freezes at 32° Fahrenheit (F) and boils at 212°F (at sea level). In fact, water's freezing and boiling points are the baseline with which temperature is measured: 0° on the Celsius scale is water's freezing point, and 100° is water's boiling point. Water is unusual in that the solid form, ice, is less dense than the liquid form, which is why ice floats.Water has a high specific heat index or capacity. This means that water can absorb a lot of heat before it begins to get hot. This is why water is valuable to industries and in your car's radiator as a coolant. The high specific heat index of water also helps regulate the rate at which air changes temperature, which is why the temperature change between seasons is gradual rather than sudden, especially near the oceans.
- Big Idea. Water has unique properties. About 97 percent of all water is in the oceans. Salt water or seawater has characteristics similar to fresh water with some noticeable differences because of the salts that are dissolved in water.
- Grade Level. Middle.
- Time. Up to three 45-minute periods.
- Content Standard. NSES Physical Science, properties and changes of properties in matter.
- Ocean Literacy Principle. (1e) Most of Earth's water (97%) is in the ocean. Seawater has unique properties: it is saline, its freezing point is slightly lower than fresh water, its density is slightly higher, its electrical conductivity is much higher, and it is slightly basic.
Materials
Station 1 (Boiling Point). Distilled water, seawater (or "Instant Ocean" mix from a pet store that sells salt water fish), isopropyl alcohol (optional), hot plate, 3 flasks with rubber stoppers that hold a thermometer, 3 thermometers that can measure from -10°C to 110°C, graph paper.Station 2 (Freezing Point). Distilled water, seawater (or "Instant Ocean" mix), isopropyl alcohol, 3 thermometers that can measure from -10°C to 110°C, 3 large test tubes with a one hole fitted stoppers, 3 Pyrex beakers, dry ice chunks, gloves, graph paper.
Station 3 (Heat Capacity). Hot plate, 2 flasks (same size), 2 thermometers, bucket of ice water, stop watch.
prop_fresh_sea.pdf (2.1 MB)